What is a clinical trial?
A clinical trial is a form of scientific research aimed at evaluating new diagnostic or therapeutic methods. Such methods may involve new drugs or therapies, a new dosage or a combination of different therapies, such as administration of chemotherapy at the same time as radiotherapy, and/or hormone therapy, and/or immunotherapy, etc.
In the case of cancer, the aim of clinical trials is to document more effective treatments with the least possible side effects, but to also improve the quality of life compared to existing therapeutic treatments. Only volunteers participate in them.
Patients with malignancies can benefit in the form of additional treatment options and/or by contributing to the discovery of new biomarkers in cancer, as well as to the application of new technology analytical methods with the ultimate goal of an individualized therapeutic approach.
There are two types of clinical trials. Interventional and non-interventional. Interventional trials are those which involve the administration of an investigational medicinal product (drug) (IMP) and the non-interventional clinical trials involve translational research and recording without the administration of a research drug (IMP).
All clinical trials conducted worldwide are registered in a database (ClinicalTrials.gov). It is accessible to everyone with further information on any specific clinical trial.
After a research drug has gone through the preclinical phase of testing on either cell lines and lab animals in a laboratory controlled environment, it is then applied and evaluated in humans taking into account the benefit/risk ratio.
Phases in clinical trials until final approval of a new drug
Clinical Trials in Humans consist of four phases:
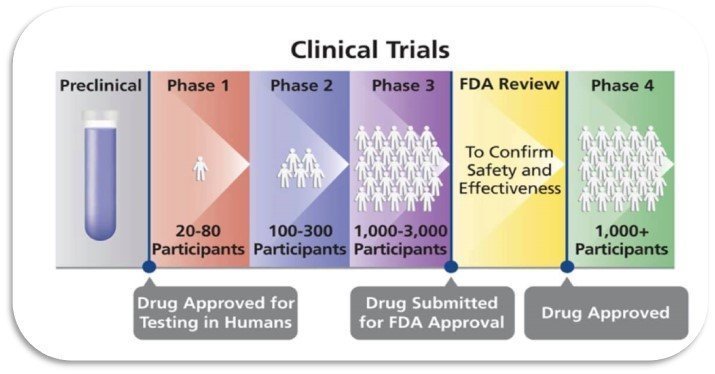
Phase I: testing of new treatments in humans for the first time. Only a small number of people receive the experimental treatment. Main goal: to collect information, evaluate and determine a safe treatment dose for human use. Side effects are reported and monitored closely.
Phase II: More people are tested for a longer period of time. Main goal: to find out if the treatment works for people with a certain type of cancer. Side effects continue to be monitored closely and any new ones reported. If enough people participate and the benefits outweigh the side effects, phase III can begin.
Phase III: Testing of treatment is carried out in a much larger group of people and usually last loner than phases I and II. Main goal: The experimental treatment will be compared against a standard treatment, or two different experimental treatments may be compared to each other. Side effects are monitored over a longer period of time and reporting of any new ones continues.
Phase IV: Treatment tested in very large number of people after the drug or device has been approved by the FDA. Main goal: To collect information regarding how treatments are used and monitor side effects, safety and effectiveness over longer periods of time in a very large group of people.
How are clinical trials conducted?
Clinical trials involving patients are conducted under strict scientific and ethical guidelines applied worldwide and are reviewed before receiving final approval from international organizations such as the FDA - U.S. Food and Drug Administration (fda.gov) and the EMA - European Medicines Agency | (europa.eu).
In Cyprus, clinical trials concerning medicinal products for human use are carried out only after submission for review and approval by the Cyprus National Bioethics Committee (CNBC) (established in 2005 under the Bioethics Law 150(I)/2001) and by the Pharmaceutical Services Department of the Ministry of Health.
Each clinical trial conducted at the Bank of Cyprus Oncology Centre has been evaluated and approved by the Centre’s Research Committee prior to its submission to the regulatory authorities of the Country (Cyprus National Bioethics Committee and Pharmaceutical Services).
In addition, a regular on-site inspection of patient data for quality and protocol compliance is carried out by the contractor organization (pharmaceutical company or international contract research organization), which is responsible for monitoring all the work for the trial, in order to preserve its reliability.
These mechanisms are in place for:
- the protection of the people taking part in the trials (respect for the value of a human being)
- the supervision and monitoring of trials in order to comply with Good Clinical Practice (starting from the Nuremberg Code and until recently the revised Helsinki Declaration)
- safeguarding the highly sensitive personal data of the individuals involved.
What are inclusion and exclusion criteria?
Each clinical trial carries specific inclusion and exclusion criteria that must be met in order for a patient to enter a trial. Based on whether these are met or not, the doctor will decide which patient is eligible for a trial. These criteria are mainly based on age, gender, type and stage of the disease, haematology and biochemical blood test results as well as previous history of treatment and diseases and define the characteristics of the study population.
The participation of a patient in a clinical trial is exclusively his/her own decision and can refuse or withdraw from a trial at any time he/she decides without affecting his/her treatment, even after his/her inclusion in the study.
If the patient is considered eligible for a trial, a patient consent and information form is provided.
What is informed written consent?
Patients participate voluntarily and only after written and oral information and consent. No patient can enter a clinical trial without signing an "informed consent form". During the presentation and explanation of this form, the doctor or other researcher will explain to the patient the purpose of the study, the procedures involved and the potential risks and benefits of participation.
Patients who meet all the inclusion and none of the exclusion criteria of a trial and are deemed suitable for participation by their doctor, will receive a code that will be study specific and will be used from the moment of their trial entry, where they will protect their identity and personal data in line with the European Data Protection Regulation (GDPR). This way, all information is encoded throughout the study.
Clinical Trials at the Bank of Cyprus Oncology Centre
Since its establishment, the Centre has demonstrated significant clinical research activity, which has always been a high priority. Within the Centre, the Clinical Trials Unit (CTU) has been conducting clinical trials for over 23 years (since 1999).
Our Centre participates in clinical trials at a national and global level ranging from phases I-IV.
Patients may be informed by their treating doctors about clinical trials available, that are suitable for them, provided that they meet the selection criteria.
The success of clinical trials depends on the voluntary involvement of patients. At the Centre, patients are actively involved in decisions related to their health and have the opportunity to receive a treatment before it becomes widely available (clinical trial).
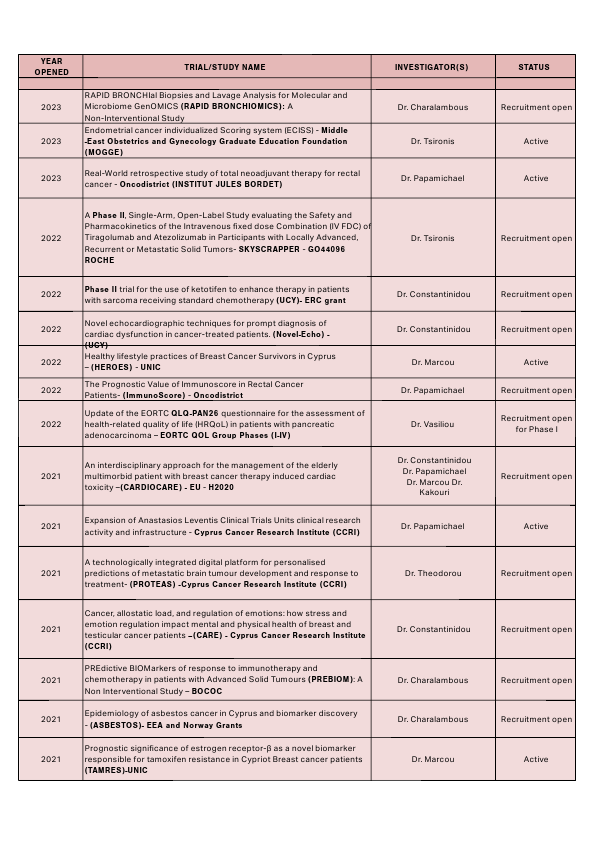
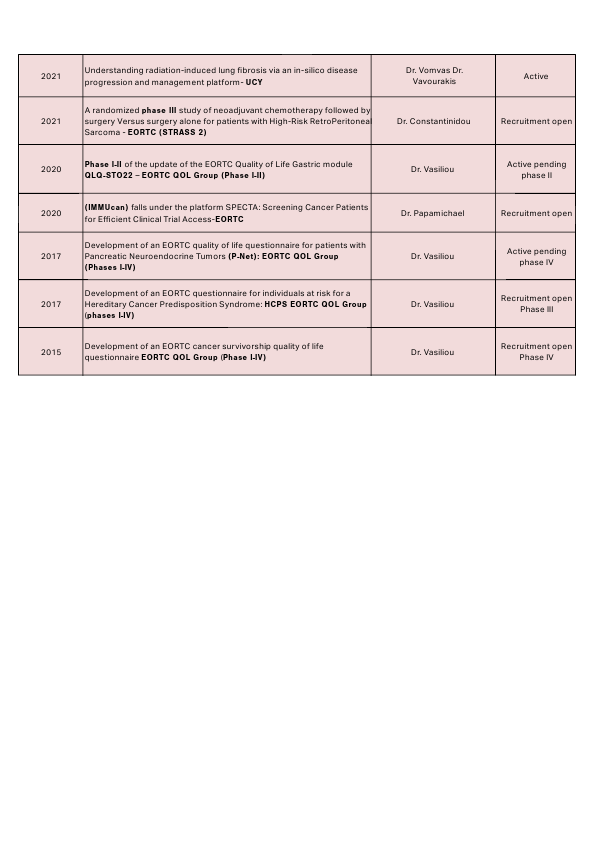
Clinical trials currently available at the Bank of Cyprus Oncology Centre can be found here:
OUR TEAM
Clinical Trials Research nurses
Ioannis Stylianou
ioannis.stylianou@bococ.org.cy
Loukia Georgiou
Research Fellows
Antri Demetriou
Dr. Alexia Alexandraki
alexia.alexandraki@bococ.org.cy
Georgia Karaoli
Dr. Elisavet Fotiou
elisavet.l.fotiou@bococ.org.cy
